Which Describes the Oxidizing Agent in a Chemical Reaction
Oxidizing agents are substances that gain electrons in a chemical reaction. Oxidizing agents can be defined in two different ways.

How To Find The Oxidizing And Reducing Agent Youtube
2C8H18 25O2 16CO2 18H2O 6.
. 2Cl- F2 2F- Cl2 b. This results in the reduction of oxygen. The reducing agent and the oxidizing agent.
Correct answer to the question Which describes the oxidizing agent in a chemical reaction. Which describes the oxidizing agent in a chemical reaction. It is typically in one of its lower possible oxidation states and is known as an electron donor.
An agent of something is an entity that carries out the required task. Ag and Cu During a redox reaction the term reduction refers to a. Which of the following is an oxidation-reduction reaction.
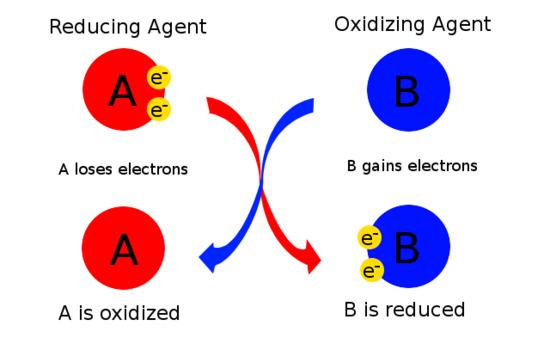
Which answer best describes what is happening in the following reaction. Oxidizing agent also known as oxidant gains electrons and it is reduced during chemical reaction. An oxidizing agent is the substance that is reduced because it gains electrons.
Oxygen shows good reactivity and easily combines with most other elements and many compounds. The substance that is oxidized because it loses electrons the substance that is reduced because it loses electrons the substance that is oxidized because it gains electrons the. An oxidizing agent also known as an oxidant oxidizer electron recipient or electron acceptor is a substance in a redox chemical reaction that gains or acceptsreceives an electron from a reducing agent called the reductant reducer or electron donorIn other words an oxidizer is any substance that oxidizes another substance.
Cl- oxidizes to Cl and F reduces to F-. Oxide-reduction reactions also called redox involve the transfer or transfer of electrons between two or more chemical species. Oxygen in elemental form is a strong oxidizing agent.
Oxidation agent is normally on its higher oxidation state since in gain electrons and be reduced. The oxidizing agent has the ability to accept or transfer those electrons. Halogens such as chlorine and fluorine Oxygen.
The presence of which reactant is the best indicator of an oxidation-reduction reaction. The oxidation agent in a chemical reaction is the substance that is reduced because it gains electrons. Lets put this in more visual terms.
In a Chemical reaction there are two species- one is oxidizing agent and the other one is reducing agent. Which best identifies why the rusting of an iron nail in the presence of water and oxygen is an oxidation-reduction reaction. Likewise which best describes an oxidizing agent.
What describes the change in oxidation states of the following reaction. Oxidizing agent is the substance which reduces its electrons and oxidizes the other species. In these reactions two substances interact.
Describe how you would prepare each of the following aqueous solutions. Read the table below. Which reactants would lead to a spontaneous reaction.
Which describes the oxidizing agent in a chemical reaction. An oxidizing agent is a chemical substance which causes another chemical species to lose electrons. Common examples of oxidizing agents are listed below.
The oxidation state which describes the degree of. When a compound like magnesium reacts with oxygen the magnesium atoms give or donate electrons to oxygen O 2 molecules. Which describes the oxidizing agent in a chemical reaction.
The substance that is oxidized because it loses electrons. The substance that is reduced because it loses electrons. A reducing agent reductant loses electrons and is oxidized in a chemical reaction.
As per this definition oxidizing agents are the reactants that undergo reduction in. Hydrogen peroxide H 2 O 2 Potassium nitrate. The gain of electrons.
The oxidizing agent typically takes these electrons for itself thus gaining electrons and being reduced. An oxidizing agent is a reactant that removes electrons from other reactants during a redox reaction. 150 L of 0110 M NH_4_2SO_4 solution starting with solid NH_4 _2SO_4.
Chlorine Cl is the oxidizing agent because it gains an electron. 1 Get Other questions on the subject. Now we discuss the movement of electrons.
An oxidizing agent is. Reducing Agent is the. In order for it to oxidize something else it must itself be reduced.
Because of this action oxidizing agents are also known as electron acceptors. Defining oxidation as the increase in oxidation state eg from 1 to 2 we see that charge is becoming more positive. As an electron acceptor They are chemical substances whose atoms remove at least one electron from another atom in a chemical reaction.
Which describes the oxidizing agent in a chemical reaction. It is the oxidizing agent. Oxidation means the loss of electrons the loss of a hydrogen atom or the addition of an oxygen atom.
Which describes the oxidizing agent in a chemical reaction. Updated on April 04 2020. Thus an oxidizing agent must oxidize.
ZnSs 2O2g mc011-1jpg ZnSO4s.

Which Substance Is The Oxidizing Agent In This Reaction 2cuo C 2cu Co2 In 2022 Oxidizing Agent Substances Agents

8 2 Oxidizing And Reducing Agents Chemistry Libretexts
In A Redox Reaction If One Reactant Is The Oxidizing Agent Must The Other Reactant Be A Reducing Agent And Vice Versa Quora
No comments for "Which Describes the Oxidizing Agent in a Chemical Reaction"
Post a Comment